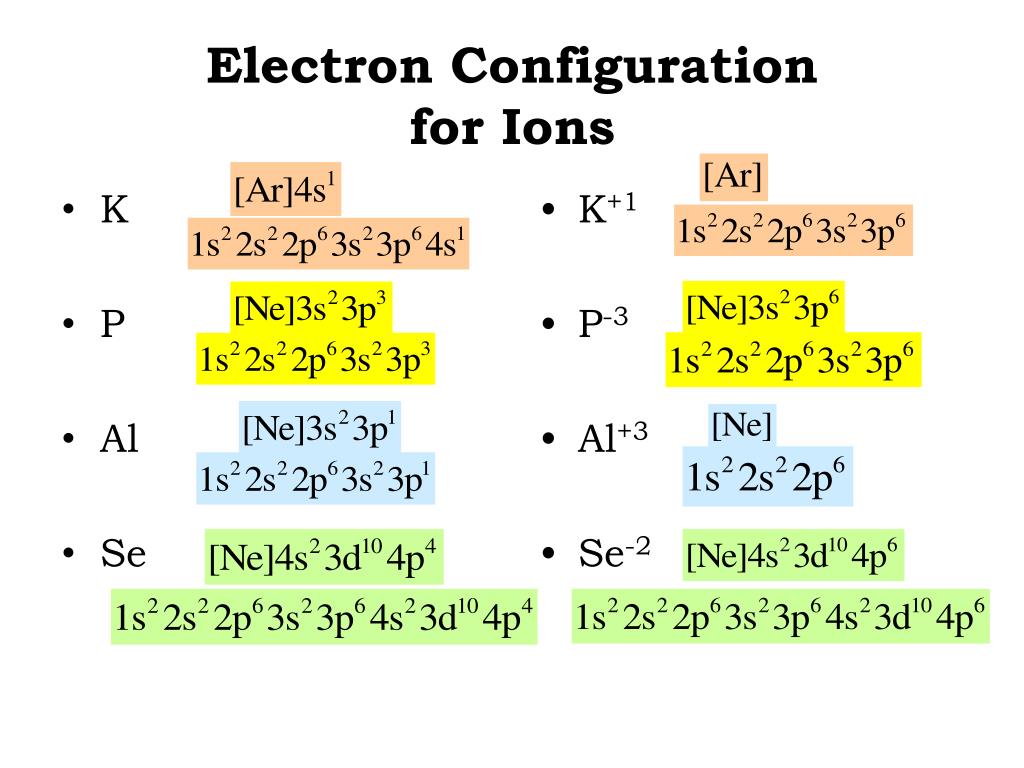
K+ Electron Configuration (Potassium Ion) (With images) Electron
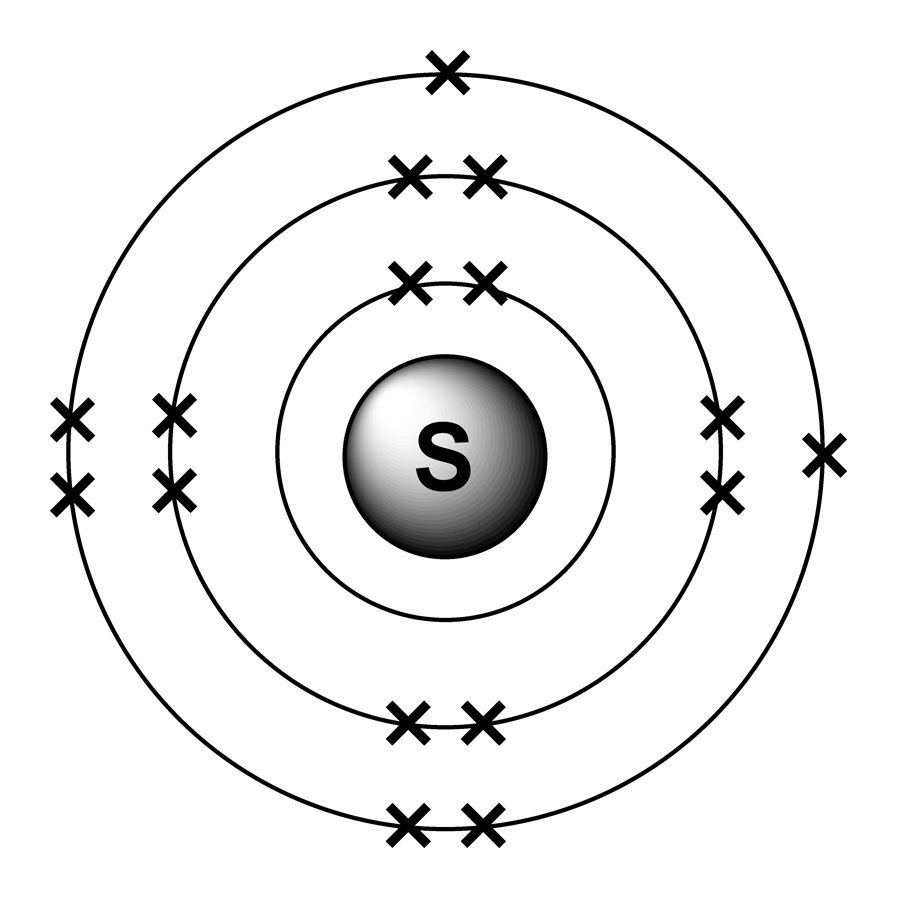
Sulfur Table of Elements by Shrenil Sharma
Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle) Electron configuration through orbital (Aufbau principle) Potassium (K) atom electron configuration (Bohr model) Electron configuration through orbitals follows different principles.
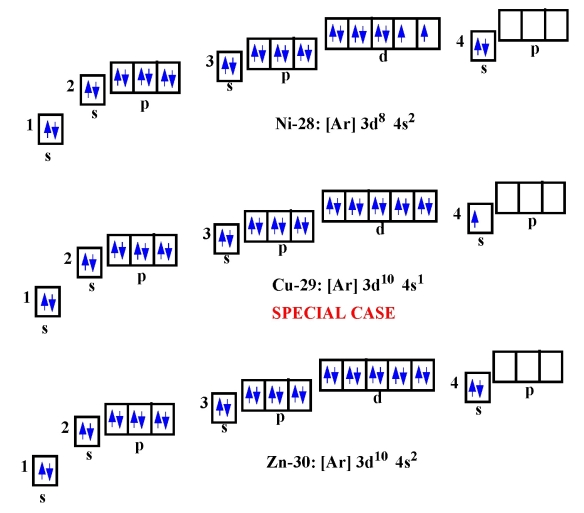
Electron Configuration For K slidesharetrick
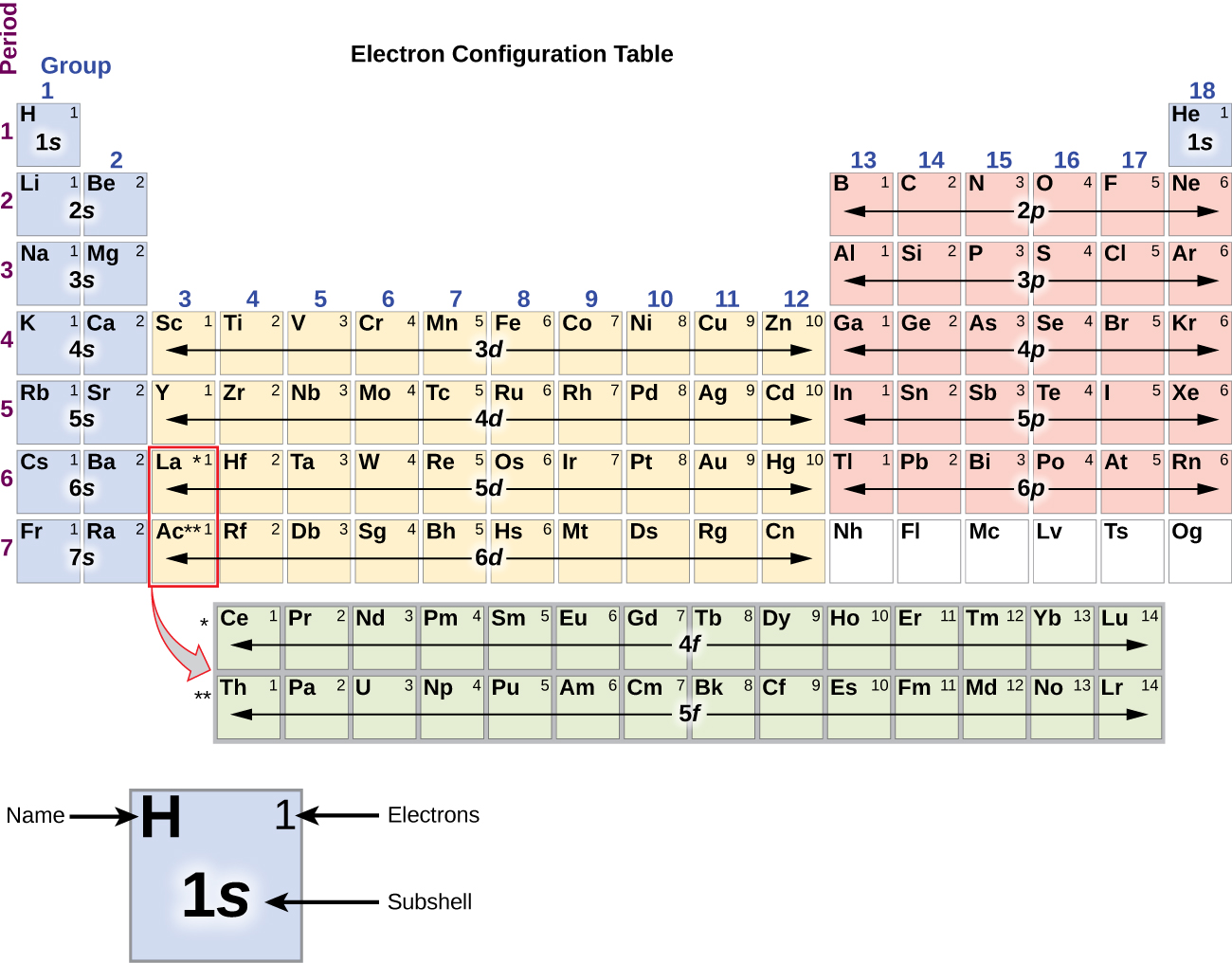
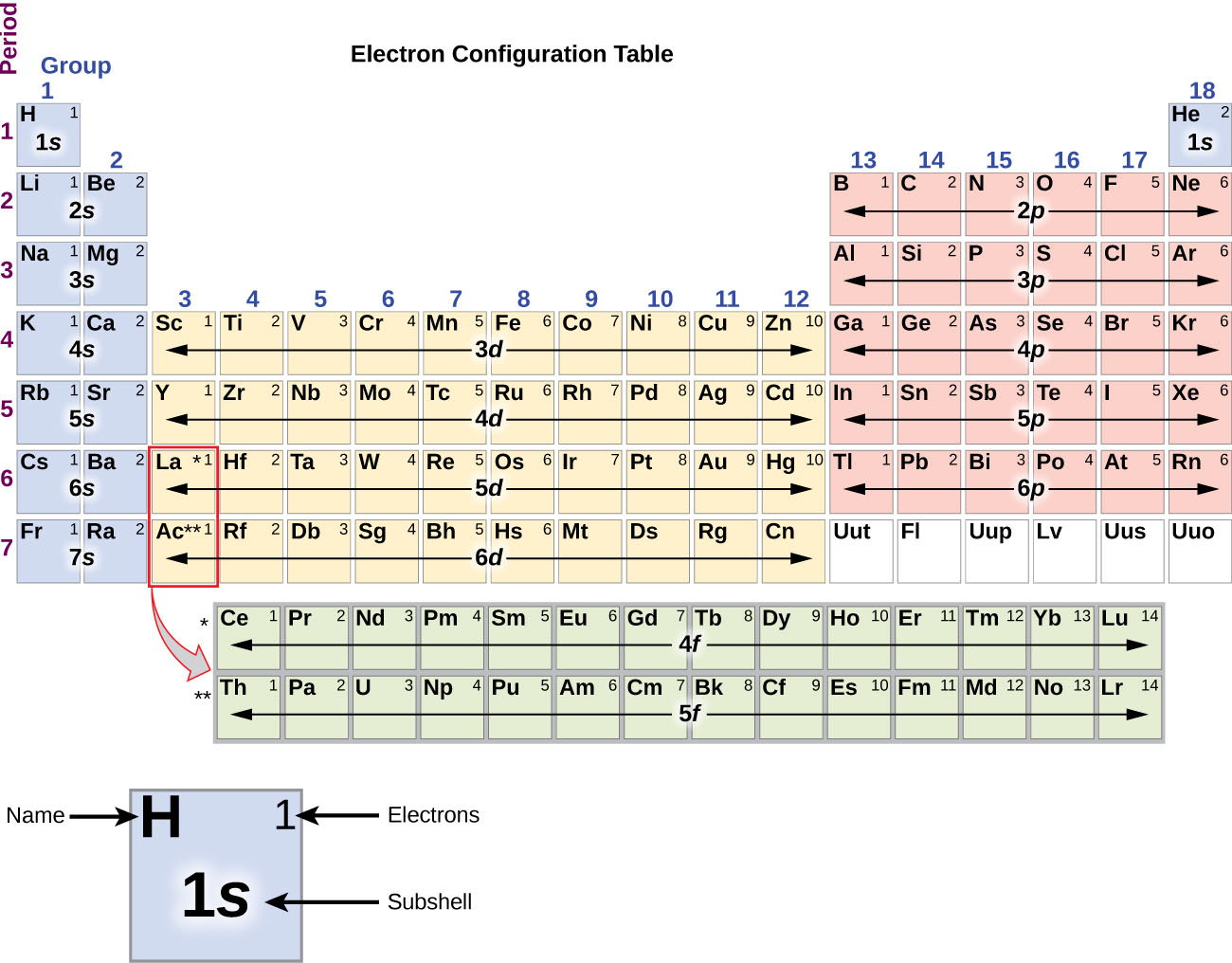
The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.

K+ Electron Configuration (Potassium Ion) (With images) Electron
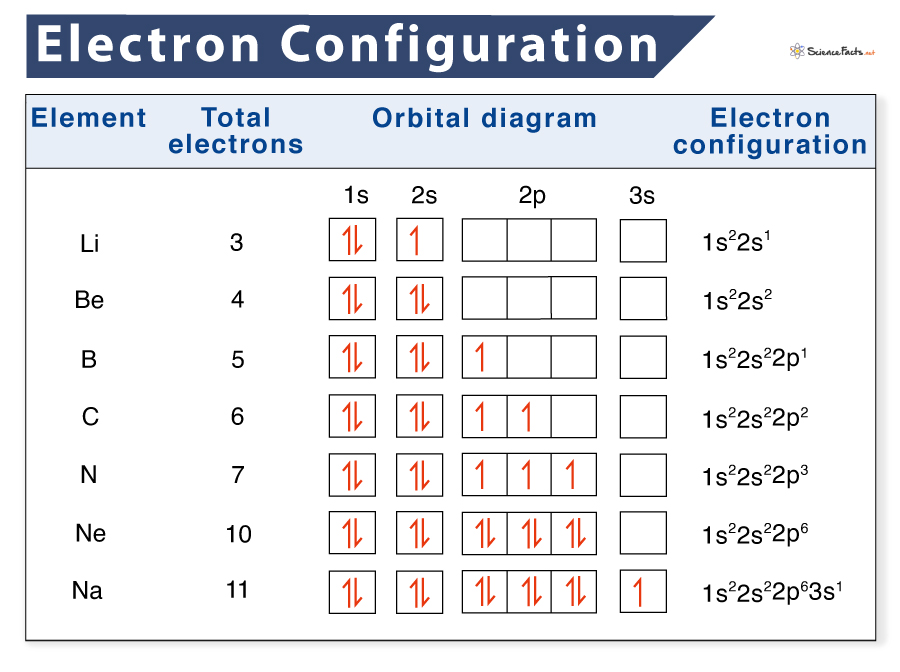
Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted
Electron Configuration For K slidesharetrick
Referring to either Figure 2.6.3 2.6. 3 or 2.6.4 2.6. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.
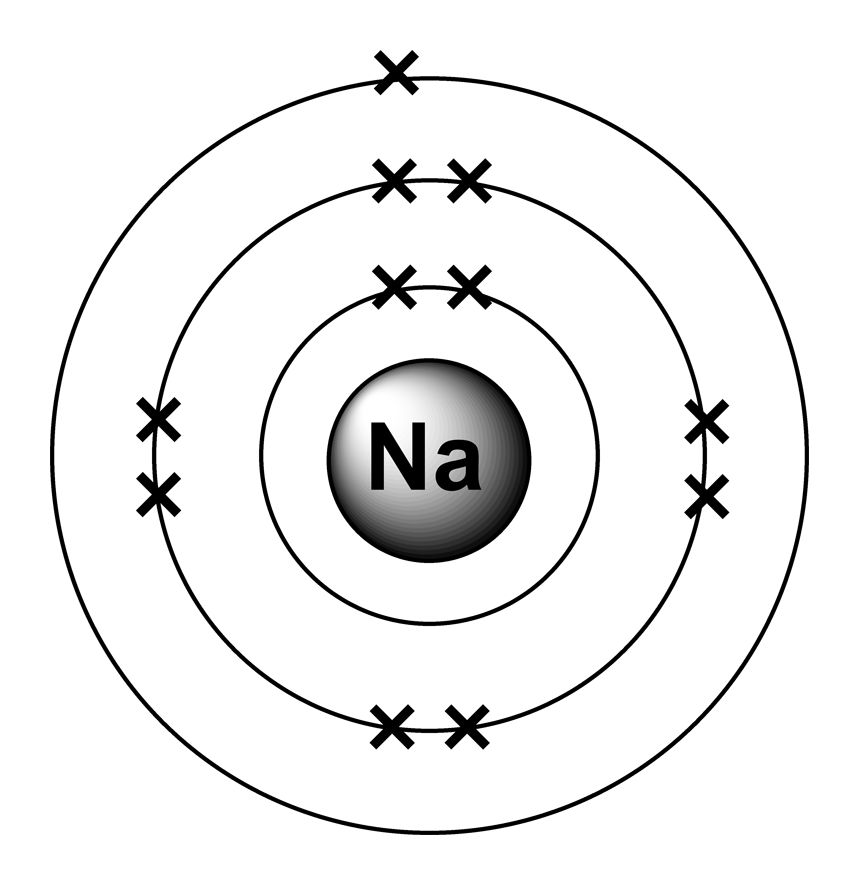
Sodium Table of Elements by Shrenil Sharma
Find the electron configuration for Potassium (K) chemistNATE 252K subscribers Subscribe 188 Share Save 15K views 3 years ago Potassium is the FIRST element in the FOURTH row of the table. So.
Silicon Electron Configuration How Many Unpaired Electrons Ellis
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n - 2) f, the ( n - 1) d, and the ns subshells. There are two inner transition series:
M7Q7 Electron Configurations, Orbital Box Notation Chem 103/104
What are Electron Configurations? The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.

Electronic configuration Definition, Orbitals, & Facts Britannica
In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll put all 19 electrons in orbitals around the nucleus of the Potassium atom.

Electronic Structure of Atoms (Electron Configurations) Chemistry
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

1.5 Electronic Structure of Atoms (Electron Configurations)
Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: Notable Reactions with Phosphorus. Potassium reacts so violently with water that it bursts into flame. The silvery white metal is very soft and reacts rapidly with the oxygen in air. Its chemical symbol is derived from the Latin word kalium which means "alkali". Its English name is from.
Electron Configuration
A step-by-step description of how to write the electron configuration for Potassium (K). In order to write the K electron configuration we first need to kno.

code golf Electron Configuration Diagrams Code Golf Stack Exchange
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Ne 3s23p3 Is the Electron Configuration of an __________ Atom Baker
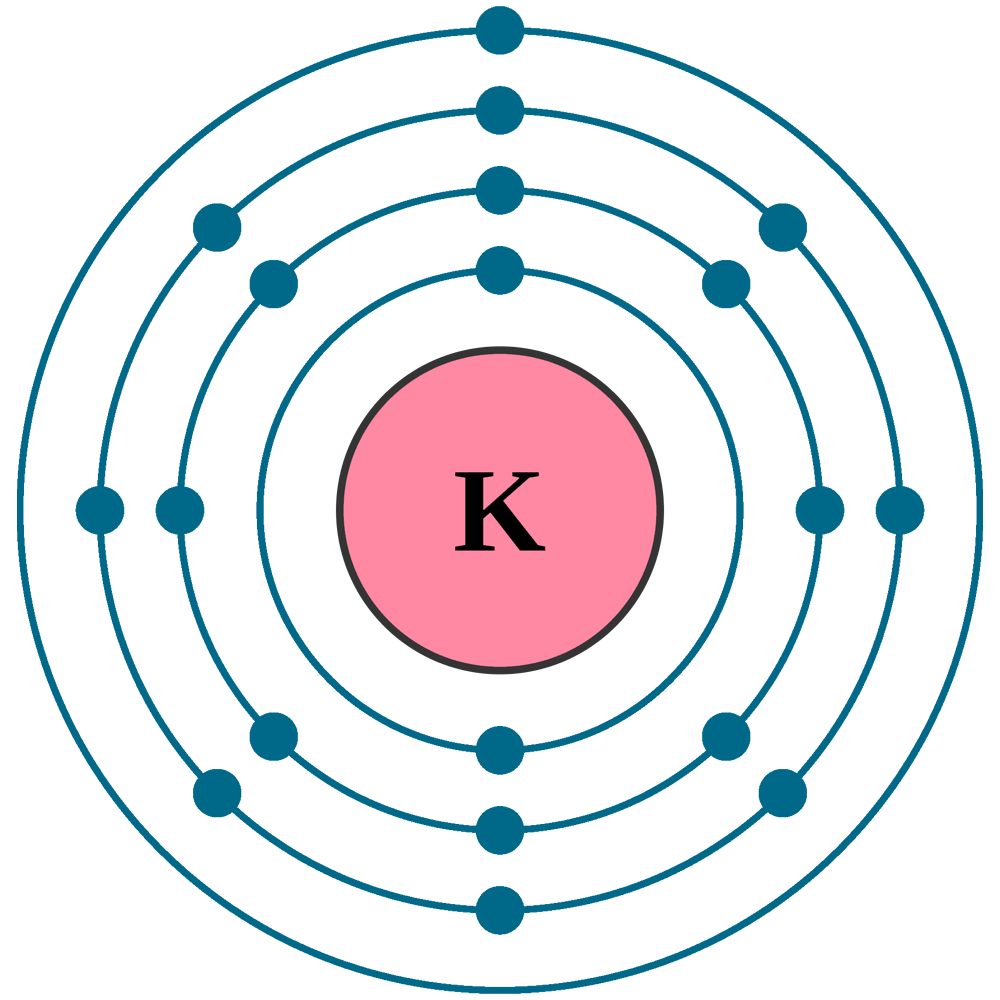
The total electron configuration for potassium is K = 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 This simplifies to a noble gas notation of K = [Ar] 4s^1 We use the symbol for argon (Ar) because it is the last noble gas in the period (row) above potassium on the periodic table.
Potassium K (Element 19) of Periodic Table Elements FlashCards
In this video we will write the electron configuration for K+, the Potassium ion. We'll also look at why Potassium forms a 1+ ion and how the electron config.
PPT Chemical Bonding PowerPoint Presentation, free download ID3249254
Potassium Electron Configuration: Potassium is a chemical element. Its symbol is K that is taken from Neo-Latin kalium. The atomic number of potassium is 19. Firstly it was isolated from potash and the ashes of plants, from where its name is derived. In the periodic table. Potassium (K) is one of the alkali metals.
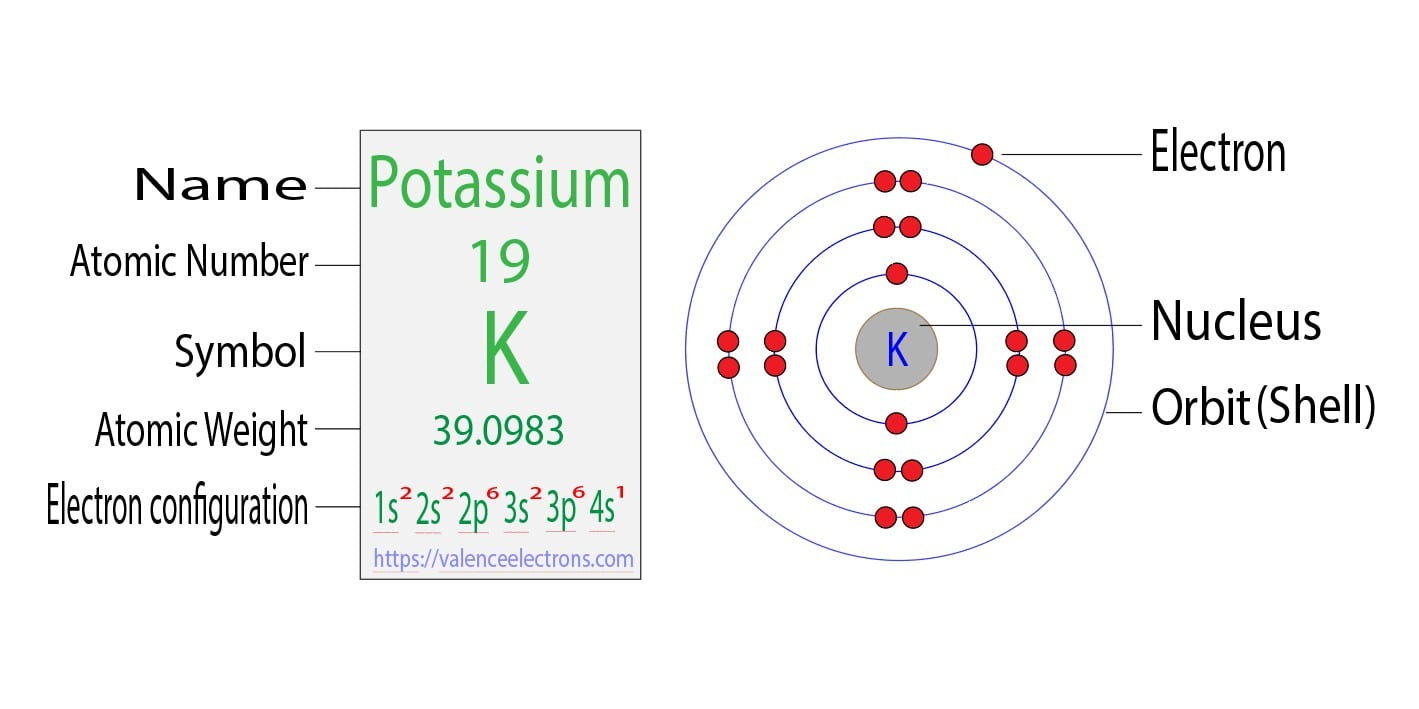
Bohr Diagram For Potassium
The Potassium has 19 electrons out of which 18 are from the argon gas configuration and the remaining one electron is filled in 4s. Potassium unabbreviated electron configuration In the Unabbreviated electronic configuration, the ground state electronic configuration of the potassium atom is as follows: 1s 2s 2p 3s 3p 3d 4s.